The cannabis plant has fascinated many civilizations, societies, and individuals through the centuries with its unique properties, and many have learned how to benefit from these, finding a variety of therapeutic and industrial uses of the plant that, in turn, enhanced domestic economies worldwide. In Thailand, cannabis plants and their derivatives have been used since ancient times as treatment for many diseases, and the plant forms a key ingredient in many Thai traditional medicinal remedies. However, over the past few decades, cannabis usage was seen to change in a way that became incrementally more abusive, resulting in outright prohibition in almost all countries. Thailand was no exception, and in 1979 the Thai government officially enacted the Narcotics Act forbidding the use of cannabis and listing cannabis plants and their derivatives—most notably marijuana (cannabis with psychoactive properties) and hemp (cannabis with limited or no psychoactive properties)—as category 5 narcotics (i.e., prohibited substances).
Despite these restrictions, many Thais continued to use cannabis illegally, and some urged the government to legalize personal and commercial use of cannabis plants and their derivatives. Eventually, some in the Thai government agreed that it was time to consider steps toward legalization, As a result, the government has been taking action to delist cannabis plants from the list of prohibited narcotics since 2018, when a regulation allowed the cultivation of hemp for industrial and non-commercial purposes, such as household cooking and research and development.
The next significant step came in February 2019, when the Narcotics Act (No. 7) was amended, legalizing medical marijuana within certain limitations. In national elections the following month, the Bhumjaithai political party, whose election campaign included a pledge to decriminalize and legalize cannabis plants, won substantial support in parts of the country and chose to join the coalition government, with the Bhumjaithai party leader assuming control of the Ministry of Public Health (MOPH). Since then, the government, with cooperation from the MOPH and the Food and Drug Administration (FDA), has been working to reclassify cannabis products and to issue new regulatory pathways to accommodate these new “cash crops” to boost the domestic economy. Subsequently, a notification was issued in August 2019 that reclassified certain modern drugs, cosmeceuticals, nutraceuticals, cosmetics, and food containing hemp out of the scope of the Narcotics Act. At the same time, cannabis and hemp legalization were also taking a higher profile in the public sphere. For instance, the issue of cannabis legalization was raised and promoted in the election campaigns of early 2019 by one of the large political parties.
After a long interlude (caused primarily by government focus turning to the COVID-19 pandemic and other emergent priorities), a new MOPH notification was published in the Government Gazette on December 14, 2020, expanding the delisting of cannabis from the Narcotics Act to include nearly all parts of cannabis and hemp plants, as detailed in the table below.
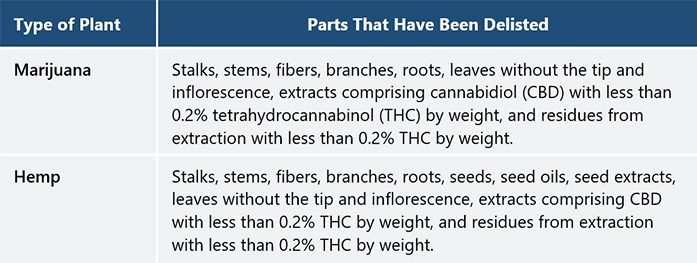
Anyone who wishes to participate in this emerging cannabis industry must be cautious and carefully review the Narcotics Act and its relevant notifications. Although many parts of the cannabis plant were delisted, there some parts remain on the narcotics list (e.g., marijuana seeds, buds, and leaves with cannabis inflorescence or complete cannabis flower head), and the limits on CBD and THC content must be strictly observed.
Importantly, this notification covers only cannabis cultivated in Thailand. Foreign participants are precluded from any participation in an approved cannabis business until a five-year ban on foreign participation—counting from the initial delisting in February 2019—has passed. This means that only the Thai government and its authorized partners have standing to apply for a commercial license until February 20, 2024, which is when foreign parties will be allowed to obtain a commercial cannabis license (subject to any extension or further imposition of restrictions). However, non-commercial licenses (e.g., for research and development) may be issued to Thai applicants.
Despite these limitations, the MOPH notification is another progressive step for driving cannabis research, development, and industry in Thailand. In addition, it delisted other plants that had been classified as category 5 narcotics, including kratom plants (a common plant in Southeast Asia with mild stimulant properties) such as Mitragyna speciosa (Korth.) Havil; opium plants such as Papaver somniferum L. and Papaver bracteatum Lindl.; and fungi of the species Psilocybe cubensis, which contain psilocybin or psilocin. Further notifications on these substances are expected to be issued soon.
The government quickly followed up on the December 14, 2020, notification by publishing another notification on December 30, establishing the criteria for filing applications for licenses to produce, import, export, distribute, and possess hemp (Cannabis sativa) in Thailand. Processing of these applications by the FDA opened on January 29, 2021, for interested Thai citizens and entities under Thai law. Industry operators are now waiting for the Thai FDA’s final promulgation of its own regulations for cannabis licensing to support this development.
Parties interested in entering into this emerging industry should closely monitor the ongoing development of the regulatory regime for cannabis plants in Thailand, as this regime is solidifying rapidly. Those who stay apprised of the situation and remain ready to act when opportunities arise will be able to take full advantage of the new regulatory changes, and in the process will help strengthen a nascent industry in Thailand.





