A new royal decree in Thailand (Royal Decree Re: Licensee to Pay the License Renewal Fee in Lieu of a Grace Period When Submitting a License Renewal Application B.E. 2564) does away with the current red tape associated with renewing certain marketing authorization and business licenses. Under the new royal decree, there is no longer any need to submit renewal applications for eligible licenses or to wait for approval from the relevant authority. Instead, the licenses will be automatically renewed upon payment of renewal fees. There are 31 eligible licenses listed in the royal decree, with a focus on licenses for hazardous substances and cosmetics.
The royal decree on license renewal was published in the Government Gazette in May 2021 and will come into force on November 22, 2021. Although the new royal decree has not yet come into force, Thailand’s Food and Drug Administration (FDA) has already begun renewing licenses for hazardous substance licenses and cosmetics notification receipts via the new procedure. These licenses and notification receipts are detailed below:
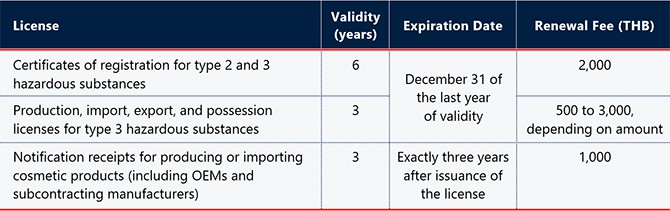
(Note: Hazardous substances for household use and public health are classified into four types according to their risk. Products containing type 2 and 3 substances are a higher risk than type 1 and must be registered with the FDA. Type 4 substances are prohibited.)
Procedural Guidance
The licenses for hazardous substances in the table above should be renewed in the two months preceding their expiry date, while the notification receipts for cosmetics should be renewed in the six months before their expiry date. Renewed licenses are valid for another six or three years (i.e., matching the original validity period).
Under the new royal decree on license renewal, eligible licenses for hazardous substances and cosmetics can be renewed by notifying the FDA via its e-submission system. The license holder will then receive a payment order for the renewal fees, which can be paid through various channels (e.g., payment service points, banks, offices of the relevant authorities, and electronic banking). Upon payment, the license will be renewed automatically and the license holder can download a new license for the cosmetic product or a license attachment for the hazardous substance. It can be used instead of or attached to the old license, and there is no need to change the FDA number.
Other Considerations
The new renewal procedure under this royal decree will greatly reduce the time and effort businesses spend seeking license renewal. Nonetheless, this simpler renewal procedure could also be cause for concern due to the lack of verification or approval in the process. The relevant authority may find it difficult to verify and ensure that businesses are operating properly under relevant laws, which could lead to safety issues for consumers and the general public.
For instance, this streamlining of the license renewal process bears some similarity to circumstances surrounding the recent hazardous substance factory explosion in Samut Prakan Province. In the days after the explosion, the latest amendment of the Factory Act B.E. 2562 (2019) was criticized by the public because it removed license renewal requirements, instead stipulating that factory operation permits no longer had an expiry date—a change from the previous requirement to renew the permit every five years. This change meant that factories no longer had to be verified every five years when it was time to renew the permit. Without verification and monitoring from the relevant authority, it is difficult to confirm factory safety and compliance with the relevant laws and regulations.
Similarly, this latest royal decree on license renewal outlines an easy renewal procedure (automatic approval) without verification or approval from the relevant authority. This lack of regulatory verification and approval could lead to heightened safety concerns for employees, consumers, and the general public. It seems that it is now up to businesses themselves to verify their own regulatory compliance and to be aware of any changes that require a license amendment before renewal. It is the responsibility of the business to ensure that its products are safe and in full compliance with laws and regulations. Therefore, this license renewal procedure under the new royal decree eases bureaucratic burdens on businesses but also serves as a reminder of their essential role in protecting consumers and the public interest.






